Research Article - (2023) Volume 14, Issue 1
Use of Polymers Showing Synergistic Effect to Increase the Release of Tolmetin Sodium in Topical Gel
Gaikwad Sakshi*, Patil Mukesh, Bhosale Siddhi, Naiknaware Tushar and Jain AshishAbstract
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) are the most widely prescribed drugs worldwide. They act as analgesic, anti-pyrectic and anti-inflammatory agents. This class of drug reduces pain, inflammation, lower down body temperature. They act by slowing the formation of prostaglandins and also blocks cyclooxygenase enzyme. This reduces inflammation. The major drawback is considered to be its gastrointestinal ulcers and bleeding, heart attack and kidney disease. Tolmetin sodium is used for its anti-inflammatory activity. Present therapy of Tolmetin sodium indicates the oral route of administration. Side effects of tolmetin sodium that have been recorded include gastrointestinal ulcers and hepatotoxicity. To overcome the side effects of tolmetin sodium, topical administration can be preferred over oral administration.
Skin is a major barrier for topical dosage form. It affects the systemic bioavailability of drug. Release of tolmetin sodium from topical gel can be increased in various ways. The activity of the drug in topical formulation with carbomer base is found to be highest as compared to that of Hydroxypropyl methylcellulose base. Polymers showing synergism can be used to increase release rate which increases the potency of Tolmetin sodium in gel formulation. This enhancement of release rate increases the bioavailability of the drug in the systemic circulation.
Keywords
Tolmetin sodium, Carbomer release, Analytical method, Tolmetin gel, Drug release
Introduction
Topical drug delivery system
The system in which the drug is carried through the skin is called as a topical drug delivery system. Skin is considered a major barrier in topical drug delivery systems. Topical formulation is applied externally as well as internally. The external preparations are applied on the cutaneous layer whereas internal topical preparation is applied on mucous membrane or cavities (Sharadha M, et al., 2020).
Advantages:
• Convenient
• Ease to apply
• Exclude first-pass metabolism
• Patient compliance
• Self-administration
• Avoid gastric and intestinal incompatibility
• Site-specific action (Sharadha M, et al., 2020)
Skin
The intricate network of epidermis, dermis and hyper dermis forms body’s initial barrier against pathogenesis. The thickness of skin varies depending on the region due to differences in demand and epidermal layer (Yousef H, et al., 2017).
Layers of epidermis
The layers of epidermis are mentioned below (Gilaberte Y, et al., 2016) (Figure 1)-
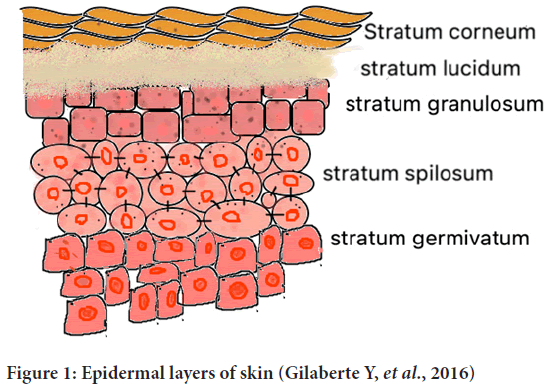
Figure 1: Epidermal layers of skin (Gilaberte Y, et al., 2016)
• Stratum corneum
• Stratum lucidum
• Stratum granulosum
• Stratum spilosum
• Stratum germinativum
Dermis
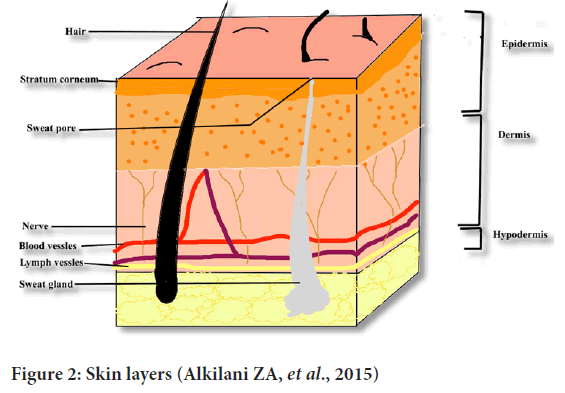
It is the thick layer of fibrous, elastic tissue. It contains collagen, elastin and fibrillin which gives it elasticity and strength. Sweat glands, oil glands, hair follicles, nerve endings are present in this layer (Gilaberte Y, et al., 2016) (Figure 2).
Figure 2: Skin layers (Alkilani ZA, et al., 2015)
Hypodermis
Innermost layer of skin is hypodermis. Sweat glands, oil glands, hair follicles root out in hypodermis. Sebum is released into hair follicles through which it is released into the surface of the skin which protects hair and skin from moistening out like a protective step from drying (Gilaberte Y, et al., 2016).
Route of drug penetration
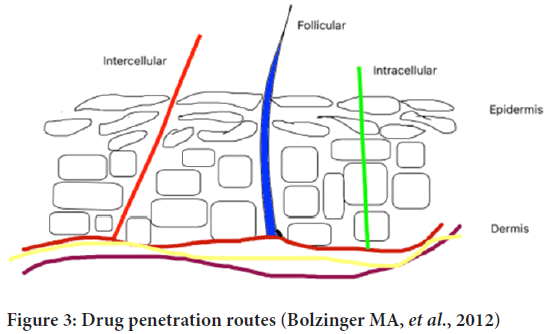
Transepidermal and transappandegeal are two possible routes of drug penetration (Figure 3). Transepidermal-In this route, molecules pass through the stratum corneum. It indulges intracellular pathway and hair follicles.
Figure 3: Drug penetration routes (Bolzinger MA, et al., 2012)
Transappandegeal-In this route, molecules enter through sweat glands and hair follicles (Alkilani ZA, et al., 2015).
Materials and Methods
Mechanism of absorption of drug through skin
Intercellular, intracellular and follicular pathways are the three main routes of drug permeation through the stratum corneum. The concentration gradient of lipid-soluble penetrant molecules is the basic mechanism for passive diffusion through the stratum corneum. Hydrophilic drugs prefer intracellular pathways through corneocytes to pass through the stratum corneum. Hair shaft and sweat glands break the continuity of the stratum corneum which provides follicular pathways but only 0.1% of the skin is covered by hair so it is a negligible pathway for drug distribution.
Drug transport inside the stratum corneum lipids is controlled by its diffusion coefficient across it along with the partition coefficient between drug formulation and stratum corneum lipids. The physical parameters like, molar mass which determines diffusion coefficient, the number of Hydrogen Bond Donors (NHD) and Acceptors (NHA) controls interaction with surface corneocytes and log P represents stratum corneum-water partition are required to consider while drug release through skin. The Lipinski’s rule of 5 has been extended to biological barriers where Mw >500, NHD>5, NHA>10 or lop P>5 (Bolzinger MA, et al., 2012).
Synergism
Synergy can be defined as the effect of the combination of agents that is more than their expected additive effect like 1+1=2. Synergism of 2 components helps to lower doses of each drug and hence less adverse effects will be seen. Super additivity and coalism are similar terms to synergy (Roell KR, et al., 2017).
Pharmaceutical gels
Gels are semisolid preparation in which one or more solute is dispersed in the solvent phase forming a network of molecules. Sol (the liquid state in which solute is dispersed) and gel state may show reversibility due to polymers used, but few polymers form a covalent bond because of which it exhibits irreversibility. The stiffness of gel is because of the interlinking chain formed by the gelling agent (Rathod HJ and Mehta DP, 2015).
Advantages of gels:
• Ease of preparation
• Adheres efficiently on skin
• Non-greasy formulation
• Tolerance to stress conditions is high (Rathod HJ and Mehta DP, 2015)
Classification of gels
Gels are classified based on the nature of the solvent used (Jeganath S and Jeevitha E, 2019).
• Hydrogels-These are based on water as solvent system
• Organogels-These are based on a non-aqueous solvent system
• Xerogels-Formulated by freeze-drying method
Based on colloidal properties:
• Organic (single phase)
• Inorganic (two phase)
Based on polymers used:
• Natural
• Semi-synthetic
• Synthetic
• Inorganic substance
• Surfactants
Formulation of gel and gel forming polymers
The linking of molecules of polymer gives a structure of the gel. Collagen, guar gum, gelatin are a few natural polymers whereas carboxymethyl cellulose, hydroxy methyl cellulose is semisynthetic, and synthetic includes Carbopol 940, poloxamer. Bentonite and cebrostearyl alcohol are some inorganic examples of polymer (Verma A, et al., 2013) (Table 1).
| Characteristics | Description |
|---|---|
| Molecular formula | C15H15NO3 |
| Molecular weight | 257.2845 |
| Chemical name | Tolmetin |
| IUPAC name | 2-[1-methyl-5-(4-methylbenzoyl)pyrrol-2-yl]acetic acid |
| Half life | 1-2 hours |
| Solubility | 222 mg/ml |
| Log P | 2.79 |
| PKa | 3.5 |
| Refractive index | 1.52 |
| Density | 1.139 |
| Melting point | 153°C |
| Boiling point | 400.53°C |
Table 1: Characteristics of tolmetin sodium (Pubchem, 2022)
Drug: Drug properties that affect diffusion through gel and movement across skin are molecular weight, lipophilicity, pH, drug tolerance i.e., it should not show zero-order release profile (Verma A, et al., 2013).
Penetration enhancers: Penetration enhancers should be non-reactive, non-toxic, non-allergic and should show solubility parameters similar to skin. It should be compatible with polymer and drug and show a quick onset of action (Verma A, et al., 2013).
Parameters for evaluation of gel are given below (Jeganath S and Jeevitha E, 2019; Verma A, et al., 2013; Patil PB, et al., 2019)-
• pH
• Viscosity
• Homogeneity
• Grittiness
• Extrudability
• Stability test
• Drug content
• Skin irritation test
• In vitro drug diffusion
• In vivo test
Results and Discussion
Overview of tolmetin sodium
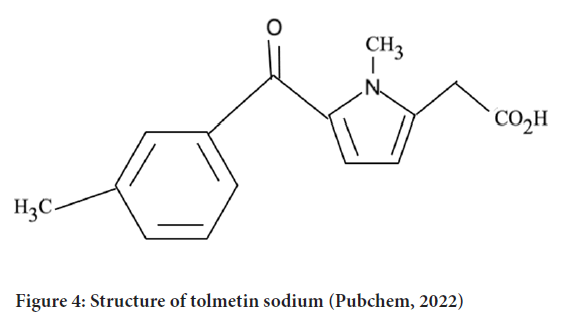
NSAIDs are used for the treatment of muscle pain, dysmenorrhea, and arthritis condition as they show antipyretic, analgesic and anti-inflammatory activity. Among the NSAIDs, tolmetin sodium, the drug containing a pyrrole ring is one of the emerging drugs that show its activity on rheumatoid arthritis, juvenile rheumatoid arthritis, degenerative joint disease and spondylosis (NLM, 2020) (Figure 4).
Figure 4: Structure of tolmetin sodium (Pubchem, 2022)
Human studies of tolmetin Sodium say that it can be an alternative to aspirin and indomethacin. The drug shows its action on inhibiting prostaglandin synthetase rather than stimulation of pituitary-adrenal. Inhibition of prostaglandin synthetase results in anti-inflammatory activity (Chen FA, et al., 2003).
Oral administration of tolmetin sodium showed minor amount of side effects including hepatotoxicity, cholestatic jaundice whereas the major amount of inflammation ulceration, bleeding on the gastrointestinal mucosa, perforation on the stomach as well as intestine (Pubchem, 2022). To overcome the side effects of present oral dosage forms. Studies have been carried out for different routes of administration including rectal, ocular and dermal. Topical administration bypasses first-pass metabolism and avoid gastrointestinal side effects. Topical administration of NSAIDS give local effect to the affected area and reduces the side effects due to systemic circulation of the drug.
Formulation of dosage form affects the penetration level of the drug from the product. The concentration of the drug in the dermis after topical administration is relatively high as compared to oral administration (Verma A, et al., 2013).
Tolmetin sodium oral dosage form overview
Tolectin DS (capsule) and Tolectin 600 (tablets) are administered orally and contain tolmetin sodium as dehydrate (Tables 2 and 3). Tolectin DS contains tolmetin equivalent to 400 mg along with 36 mg sodium and inactive ingredients like gelatin, magnesium stearate, corn starch, talc, FDandC red no. 3 and FDandC yellow no. 6 and titanium dioxide. Tolectin 600 contains Tolmetin equivalent to 600 mg along with 54 mg of sodium and inactive ingredients like cellulose, silicon dioxide, magnesium stearate, hydroxypropyl methylcellulose, corn starch, crospovidone, silicon dioxide, titanium dioxide, FDandC Yellow no. 6 and FDandC Yellow no. 10.
| Name | Dosage | Strength | Route | Manufacturers | Marketing start | Marketing end |
|---|---|---|---|---|---|---|
| Tolectin 200 tab 200 mg | Tablet | 245 mg | Oral | Mcneil pharmaceutical, division of ortho Mcneil Inc. | 31-12-1980 | 02-08-2002 |
| Tolectin 400 cap 400 mg | Capsule | 400 mg | Oral | Mcneil pharmaceutical, division of ortho Mcneil Inc. | 31-12-1976 | 02-08-2002 |
| Tolectin tab 600 mg | Tablet | 600 mg | Oral | Anssen pharmaceuticals | 31-12-1987 | 27-02-2006 |
Table 2: Branded marketed formulation of tolmetin sodium (Drugbank, 2022)
| Name | Dosage | Strength | Route | Manufacturer | Marketing start | Marketing end |
|---|---|---|---|---|---|---|
| Novo-Tolmetin capsules 400 mg | Capsule | 400 mg | Oral | Novopharm limited | 1994-12-31 | 2005-08-10 |
| Tolmetin sodium | Capsule | 400 mg | Oral | Teva | 1991-12-01 | 2014-03-31 |
| Tolmetin sodium | Capsule | 400 mg | Oral | Teva | 2012-07-04 | 2009-09-30 |
| Tolmetin sodium | Film coated tablet | 600 mg | Oral | Stat Rx USA | 2009-11-02 | NA |
| Tolmetin sodium | Capsule | 400 mg | Oral | Mylan pharmaceuticals | 1993-05-27 | NA |
| Tolmetin sodium | Film coated tablet | 600 mg | Oral | Mylan pharmaceuticals | 1994-08-30 | NA |
| Tolmetin sodium | Tablet | 200 mg | Oral | Sun pharmaceutical industries, Inc. | 2009-09-04 | 2009-09-30 |
Table 3: Generic marketed formulation of tolmetin sodium (Drugbank, 2022)
Tolectin is prescribed for acute flares as well as long-term therapy of rheumatoid arthritis, juvenile rheumatoid arthritis, osteoarthritis. Tolectin should not be administered in patients who have asthma, urticaria or those who show allergic reactions on the administration of NSAIDs. As COX-2 selective and nonselective drugs have been proved to increase the risk of cardiovascular thrombotic events, myocardial infarctions and strokes. Tolectin is not advised to give in pre-operative pain for coronary artery bypass graft surgery. Tolectin may worsen the condition in hypertension patients and may cause serious gastrointestinal side effects including bleeding, inflammation and ulceration.
Tolmetin sodium suppositories
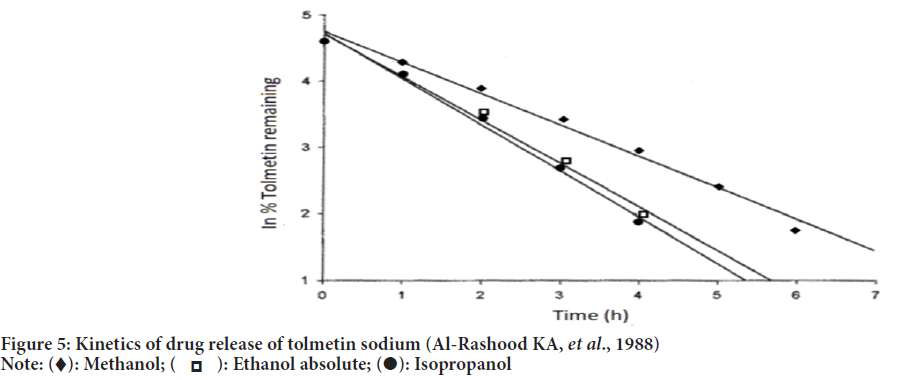
According to the studies carried out by Baloglu B and Kirkaǧaçhoǧlu O, 2002, tolmetin sodium conventional suppositories were prepared by using polyethylene glycol 400 and polyethylene glycol 4000 in various proportions as a base. Sustained-release suppositories were prepared by using eudragit L-100 which showed slow release and zero-order kinetics in dissolution studies (Figure 5). Eudragit L-100 was confirmed to be responsible for the sustained release of tolmetin sodium.
Figure 5: Kinetics of drug release of tolmetin sodium (Al-Rashood KA, et al., 1988)
Note: 
As per the studies by Ramadan AA, et al, 2018, rectal mucoadhesive hydrogel can be a better option for oral administration of tolmetin sodium. The studies were directed to formulation and evaluation of mucoadhesive rectal hydrogel of Tolmetin sodium using various proportions and concentrations of hydroxy methylcellulose, hydroxyethylcellulose, carboxymethyl cellulose and sodium alginate as polymers. The release profile showed a concentration of 72%-92.6% of the controlled release pattern of tolmetin sodium.
Overview on analytical method development of tolmetin sodium
The evaluation and selection of the most precise assay procedures to determine the composition of a drug is known as analytical method development. The analytical method development is a procedure to demonstrate the identity, characteristics, purity, potency of a drug. It includes evaluation as well as the selection of process that is precise for the determination of drug composition. The method selection of analytical procedure depends upon certain parameters like analyte physical and chemical properties, concentration and no. of samples.
Advantages of analytical method development include minimum repetition of procedures, providing surety of analyte as well as process (Doltade M and Saudagar R, 2019).
HPLC assay method for quantitation of tolmetin sodium by Chen FA, et al., 2003 satisfied the parameters like system suitability criteria, peak integrity and resolution for tolmetin sodium and indomethacin. Kinetics of tolmetin photodegradation showed first-order reaction in methanol, ethanol and 2-propanol (Al-Rashood KA, et al., 1988).
For spectrophotometric determination of tolmetin sodium, two modes that are zero-order and first-order were used to determine absorption at 325 nm and 342 nm (Table 4). The standard curve method and linear regression method were used to find out the concentration of unknown sample. Zero-order spectra peaked at 245 nm and 325 nm whereas first-order spectra peaked at 300 nm and 342 nm in the range 200 nm to 400 nm. Linear regression (r) resulted at 95% confidence limit as 0.9997 and 0.9995. Both methods used proved appropriate and suitable for the determination of tolmetin sodium.18
| Zero-order method | First derivative method | ||||
|---|---|---|---|---|---|
| Marketed dosage form | Nominal concentration | % found | % of added recovery | % found | % of added recovery |
| Tolectin capsules (200 mg) (N=6) | 1-15 | 100.42 | 99.6 | 100.28 | 100.16 |
Table 4: Determination of tolmetin sodium by zero-order and first-derivative spectrophotometry (Al-Rashood KA, et al., 1988)
Effect of penetration enhancer on release of tolmetin sodium
Release of tolmetin sodium: According to the studies carried out by Auda SH, et al., 2015 the release of tolmetin from different gel formulations for topical use showed range of release profiles. The different gel formulations were formulated using different gel bases. The studies briefly suggested the efficacy of tolmetin Sodium for local application in gel formulation.
The base types used for gel formulation were cellulose derivatives, carbopol bases, pluronic bases. The cellulose derivatives used were methylcellulose, Hydroxypropyl Methylcellulose (HPMC), CMC Na. HPMC showed a high release rate of tolmetin sodium in gel formulation whereas methylcellulose showed a low rate of drug release.
The Carbopol derivatives used were Carbopol 940 and Carbopol 934 which resulted as Carbopol 934 showed high release rate than Carbopol 940.
Release of tolmetin sodium from carbomer: Carbomer is the base of choice for gel formulation for topical preparations because of its viscosity parameter (it shows high viscosity even at low concentration) as well as it is non-toxic and exhibits antimicrobial properties to itself (Macedo T, et al., 1993). Different types of drug concentration, carbomer concentration in different composition drug release studies were carried out. Drug release comparison showed that Carbopol 941 showed fastest, 940 showed slowest and 934 were intermediate.
Increase rate of release from Carbopol 940: Glipalamide gel was prepared by direct dispersion method (Kaur DA, et al., 2014). Different batches were formulated with β-cyclodextrin, propylene glycol and oleic acid at different concentrations. β-cyclodextrin acted as a photoprotective agent whereas propylene glycol and oleic acid as penetration enhancers. Drug penetration data after 12 hours showed the result as-
All the polymers showed increased penetration of the drug.
• Oleic acid showed a greater permeation rate than propylene glycol, and propylene glycol showed more permeation rate than β-cyclodextrin.
• When compared with different concentrations of oleic acid between 5%, 10% and 15%, 10% concentration of oleic acid formulated batch showed the highest release (Table 5).
| Formulation code | Polymer used | Cumulative % drug content | Kinetics of drug release | Flux (mg/cm2/hr) |
|---|---|---|---|---|
| F1 | - | 32.2691 | Zero order | 0.997 |
| F2 | Cyclodextrin 1.1 w/w | 36.7412 | Zero order | 2.89 |
| F3 | Cyclodextrin 1.2 w/w | 46.1759 | Zero order | 1.56 |
| F4 | Cyclodextrin 1.3 w/w | 42.7764 | Zero order | 5.98 |
| F5 | Propylene glycol 15% | 52.7577 | Zero order | 7.72 |
| F6 | Propylene glycol 20% | 58.7577 | Zero order | 10.73 |
| F7 | Propylene glycol 25% | 66.9519 | Zero order | 14.39 |
| F8 | Propylene glycol 30% | 64.9049 | Zero order | 12.78 |
| F9 | Oleic acid 5% | 74.6583 | Zero order | 14.78 |
| F10 | Oleic acid 10% | 87.2711 | Zero order | 17.45 |
| F11 | Oleic acid 15% | 79.2762 | Zero order | 13.89 |
Table 5: Cumulative percent of drug permeated from formulation and flux of F1-F11 after 12 hrs (Golden GM, et al., 1987)
• The use of propylene glycol and oleic acid together showed synergistic enhancement in drug flux.
Drug flex of a solute can be stated as the mass or no. of molecules moving through a given cross-sectional area during a given period of time.
J=m/At
Where J is flux, m is mass of the compound whose flux is to be calculated and A is cross-sectional area.
Flux characterizes permeability of solute that can be transported by simple diffusion (Golden GM, et al., 1987; Drugbank, 2022).
The characteristics of penetration enhancer are similar to that of lipids in stratum corneum that allows efficient portioning with the compartments but are not similar enough to damage lipid packaging.
Conclusion
From the above studies of Tolmetin sodium release from different gel bases, the release from carbomer showed the highest activity as compared to other bases but the release from carbomer is not as good as compared to that of HPLC. The release of drug from carbomer 940 can be increased using synergistic polymers namely propylene glycol and oleic acid. The increase in the release from carbomer which shows the highest activity of Tolmetin sodium can increase the bioavailability of the drug. From the studies carried out, it can be concluded that a potent form of topical gel can be a future perspective for the treatment of arthritis and muscle pain.
Acknowledgement
I would like to acknowledge Shri DD Vispute college of Pharmacy and Research Centre for providing necessary support in the due course of the work.
References
- Sharadha M, Gowda DV, Gupta V, Akhila AR. An overview on topical drug delivery system-updated review. Int J Pharm Sci. 2020; 11(1): 368-385.
- Yousef H, Alhajj M, Sharma S. Anatomy, skin (integument), epidermis. Statpearls. 2017.
- Gilaberte Y, Prieto-Torres L, Pastushenko I, Juarranz A. Nanoscience in Dermatology: Chapter 1-anatomy and function of the skin. 2016.
- Alkilani ZA, McCrudden MT, Donnelly RF. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics. 2015; 7(4): 438-470.
[Crossref] [Google Scholar] [Pubmed]
- Bolzinger MA, Briancon S, Pelletier J, Chevalier Y. Penetration of drugs through skin, a complex rate-controlling membrane. Curr Opin Colloid Interface Sci. 2012; 17(3): 156-165.
- Roell KR, Reif DM, Motsinger-Reif AA. An introduction to terminology and methodology of chemical synergy-perspectives from across disciplines. Front Pharmacol. 2017; 8: 158.
[Crossref] [Google Scholar] [Pubmed]
- Rathod HJ, Mehta DP. A review on pharmaceutical gel. Int J Pharm Sci. 2015; 1(1): 33-47.
- Jeganath S, Jeevitha E. Pharmaceutical gels and recent trends-a review. Res J Pharm Technol. 2019; 12(12): 6181-6186.
- Verma A, Singh S, Kaur R, Jain UK. Topical gels as drug delivery systems: A review. Int J Pharm Sci Rev Res. 2013; 23(2): 374-382.
- Patil PB, Datir SK, Saudagar RB. A review on topical gels as drug delivery system. J drug deliv ther. 2019; 9(3-s): 989-994.
- LiverTox: Clinical and research information on drug-induced liver injury [Internet]: Tolmetin. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. 2012. National library of medicine (NLM). 2020.
- Chen FA, Chen CY, Chen CJ, Wu AB. Quantitation of tolmetin by high-performance liquid chromatography and method validation. J Chromatogr Sci. 2003; 41(7): 381-384.
[Crossref] [Google Scholar] [Pubmed]
- Tolmetin. Pubchem. 2022.
- Baloǧlu B, Kirkaǧaçhoǧlu O. Preparation and evaluation of tolmetin sodium conventional and sustained-release suppositories. Sci Pharm. 2002; 70(1): 77-86.
- Ramadan AA, Elbakry AM, Esmaeil AH, Khaleel SA. Pharmaceutical and pharmacokinetic evaluation of novel rectal mucoadhesive hydrogels containing tolmetin sodium. J Pharm Investig. 2018; 48(6): 673-683.
[Crossref] [Google Scholar] [Pubmed]
- Doltade M, Saudagar R. The analytical method development and validation: A review. J drug deliv ther. 2019; 9(3): 563-570. [Crossref]
- Al-Rashood KA, Mohamed ME, Al-Awadi MA, Bayomi SM. Spectrophotometric determination of tolmetin sodium in capsule dosage form. Pak J Pharm Sci. 1988; 1(2): 97.
[Google Scholar] [Pubmed]
- Auda SH, El-Rasoul SA, Ahmed MM, Osman SK, El-Badry M. In-vitro release and in-vivo performance of tolmetin from different topical gel formulations. J Pharm Investig. 2015; 45(3): 311-317.
- Macedo T, Block LH, Shukla AJ. Release of tolmetin from carbomer gel systems. Drug Dev Ind Pharm. 1993; 19(8): 887-902.
- Kaur DA, Raina AP, Singh NI. Formulation and evaluation of carbopol 940 based glibenclamide transdermal gel. Int J Pharm Pharm Sci. 2014; 6: 434-440.
- Golden GM, McKie JE, Potts RO. Role of stratum corneum lipid fluidity in transdermal drug flux. J Pharm Sci. 1987; 76(1): 25-28.
[Crossref] [Google Scholar] [Pubmed]
- Tolmetin. Drugbank. 2022.
Author Info
Gaikwad Sakshi*, Patil Mukesh, Bhosale Siddhi, Naiknaware Tushar and Jain AshishCitation: Sakshi G: Use of Polymers Showing Synergistic Effect to Increase the Release of Tolmetin Sodium in Topical Gel
Received: 02-Dec-2022 Accepted: 27-Dec-2022 Published: 03-Jan-2023, DOI: 10.31858/0975-8453.14.1.41-46
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ARTICLE TOOLS
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Psychometric properties of the World Health Organization Quality of life instrument, short form: Validity in the Vietnamese healthcare context Trung Quang Vo*, Bao Tran Thuy Tran, Ngan Thuy Nguyen, Tram ThiHuyen Nguyen, Thuy Phan Chung Tran SRP. 2020; 11(1): 14-22 » doi: 10.5530/srp.2019.1.3
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- Deuterium Depleted Water as an Adjuvant in Treatment of Cancer Anton Syroeshkin, Olga Levitskaya, Elena Uspenskaya, Tatiana Pleteneva, Daria Romaykina, Daria Ermakova SRP. 2019; 10(1): 112-117 » doi: 10.5530/srp.2019.1.19
- Dental Development between Assisted Reproductive Therapy (Art) and Natural Conceived Children: A Comparative Pilot Study Norzaiti Mohd Kenali, Naimah Hasanah Mohd Fathil, Norbasyirah Bohari, Ahmad Faisal Ismail, Roszaman Ramli SRP. 2020; 11(1): 01-06 » doi: 10.5530/srp.2020.1.01
- Manilkara zapota (L.) Royen Fruit Peel: A Phytochemical and Pharmacological Review Karle Pravin P, Dhawale Shashikant C SRP. 2019; 10(1): 11-14 » doi: 0.5530/srp.2019.1.2
- Pharmacognostic and Phytopharmacological Overview on Bombax ceiba Pankaj Haribhau Chaudhary, Mukund Ganeshrao Tawar SRP. 2019; 10(1): 20-25 » doi: 10.5530/srp.2019.1.4
- A Review of Pharmacoeconomics: the key to “Healthcare for All” Hasamnis AA, Patil SS, Shaik Imam, Narendiran K SRP. 2019; 10(1): s40-s42 » doi: 10.5530/srp.2019.1s.21
- A Prospective Review on Phyto-Pharmacological Aspects of Andrographis paniculata Govindraj Akilandeswari, Arumugam Vijaya Anand, Palanisamy Sampathkumar, Puthamohan Vinayaga Moorthi, Basavaraju Preethi SRP. 2019; 10(1): 15-19 » doi: 10.5530/srp.2019.1.3